Student Built pH Sensor

Measuring pH can inform students and scientists a lot about environmental health, as well as important chemical and biological processes occurring in ecosystems. While pH can be measured in any environment from soils to freshwater lakes, marine scientists are particularly interested in monitoring ocean pH. The concentration of carbon dioxide in the atmosphere is increasing, due to human activities. Much of the carbon dioxide enters the oceans through diffusion, which is enhanced during bubble formation generated by waves. As more and more CO2 is dissolved in the ocean waters, the pH decreases, resulting in ocean acidification. Current ocean pH monitoring programs are important to understanding how current and projected environmental changes will affect marine habitats.
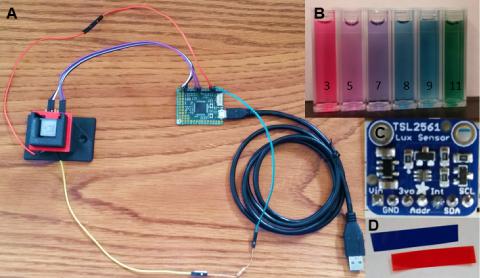
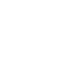
Through a new lab activity developed by graduate student Sasha Seroy and her advisor Dr. Danny Grünbaum, middle and high school students will be able to create pH sensors in the classroom. Students will have the opportunity to construct a pH sensor as an analog to how marine scientists measure pH spectrophotometrically. Using a light sensor, students detect pH by distinguishing different color solutions with an indicator dye. Red cabbage juice is a great natural pH indicator that is easily accessible and has a wide range of detectable pH values. The sensor allows students to measure transmittance of red and blue light through various standard solutions to create a calibration curve which they can then use to identify a solution of unknown pH. While the student-constructed sensors are not accurate enough to detect the small changes in ocean pH that marine scientists actively monitor, students will obtain a comprehensive introduction to the concept, which will allow them to contextualize their understanding of environmental pH measurements.